- Email: nanbei2@nanbei-china.com
The pH meter is a common laboratory instrument used to measure the pH value of a solution. It is widely used in critical fields such as water quality monitoring, food processing, and biomedicine, and is a fundamental piece of equipment for ensuring product quality, environmental safety, and production compliance.
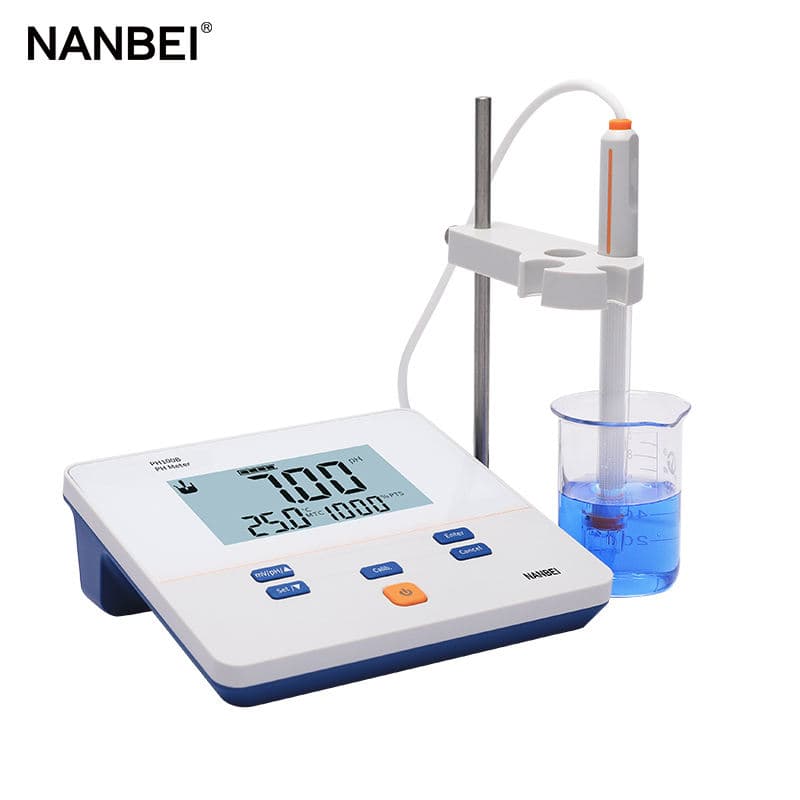
The selection of laboratory pH meter requires comprehensive consideration of factors such as accuracy and usage scenario:
I. Application Scenarios
1. Conventional Laboratories
Benchtop pH meters are typically chosen. These meters offer high accuracy and comprehensive functions, which can meet the needs of scenarios with high measurement accuracy requirements, such as chemical analysis, biopharmaceuticals, and scientific research experiments.
2. On-site or Field Testing
For on-site or field pH measurements, portable pH meters are more suitable. They are compact, easy to carry, and usually feature waterproof and dustproof properties, strong anti-interference capabilities, and are suitable for harsh on-site testing environments such as petrochemical plants and electroplating solutions.

II. Accuracy Classes
1. 0.001 Class: This is a precision pH meter, mainly used in scientific research, medicine, and other fields where extremely high pH measurement accuracy is required.
2. 0.01 Class: This is a commonly used accuracy class in laboratories, meeting the measurement needs of most routine experiments.
3. 0.1 and 0.2 Classes: These have relatively lower accuracy and are generally used for routine testing or coarse measurements where high precision is not required.