- Email: nanbei2@nanbei-china.com
A pH meter is an instrument for measuring the activity of hydrogen ions in a solution. It consists of two parts, electrode and galvanometer. The galvanometer can measure the weak potential difference in the circuit with extremely high resistance. The electrode part includes reference electrode, indicator electrode and temperature electrode.

FAQ in the use of laboratory pH meter:
1. How often does the pH electrode need to be calibrated?
It is recommended to calibrate once a day.
2. How to store the pH electrode?
When the electrode is not in use, it needs to be stored in 3mol/L potassium chloride solution (3M KCl);
3. How long is the life of the pH electrode?
Under normal use and proper maintenance, the life of the pH electrode is 1 year.
4. How long is the shelf life of pH buffer solution?
Generally, in the unopened state, the shelf life is 1 year. Once opened and used, due to the action of various molds and carbon dioxide in the air, it is easy to deteriorate. It is recommended to store the buffer solution in the refrigerator after opening, and it is better not to store it for more than one month.
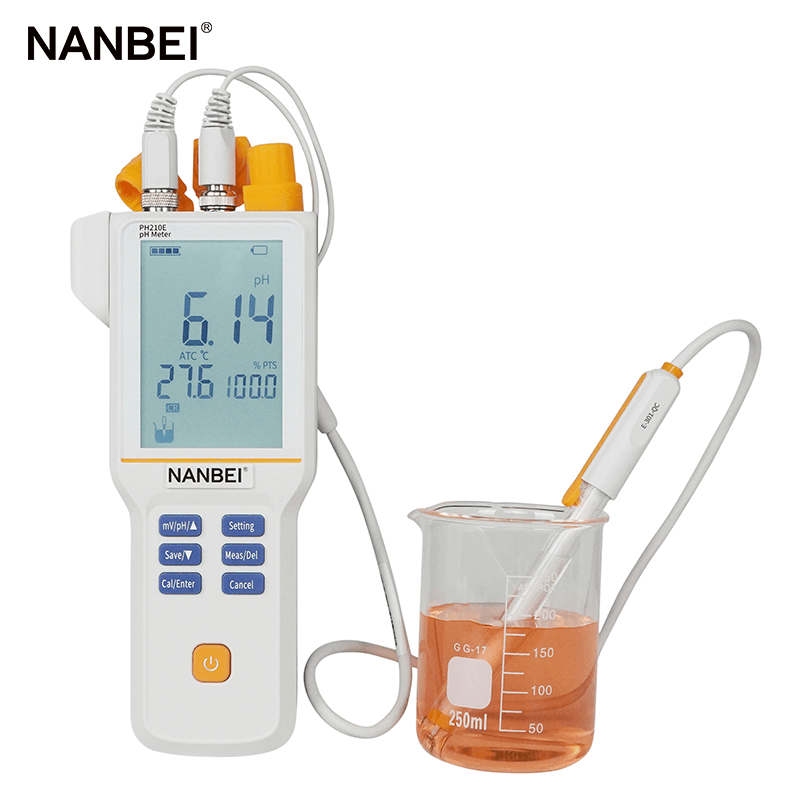
5. Why can't the pH composite electrode be soaked in deionized water for a long time?
If the composite electrode is immersed in deionized water for a long time, the KCl concentration inside the liquid junction will be greatly reduced, and the potential of the liquid junction will increase, resulting in electrode instability.
6. How to operate if the external reference solution of the pH electrode is polluted?
For rechargeable electrodes, you can prepare a new KCl solution and add it in, pour it out after the first and second additions, so as to clean the inner cavity.
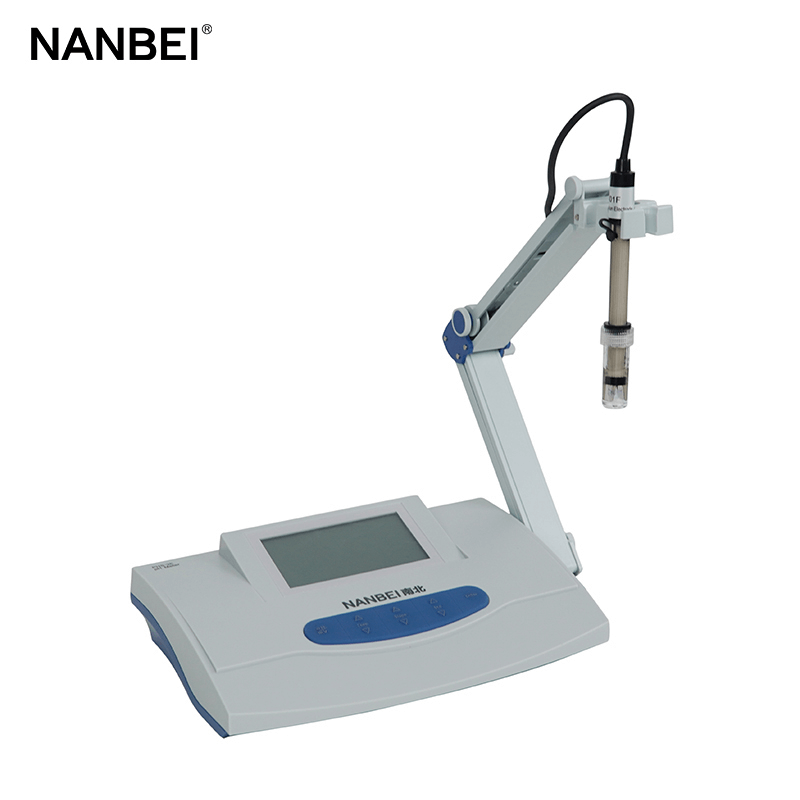
Acidimeter, also known as pH meter tester, is a commonly used instrument, mainly to precisely measure the pH value of liquid medium, which is widely applied in industry, agriculture, scientific research, environmental protection and other fields.
